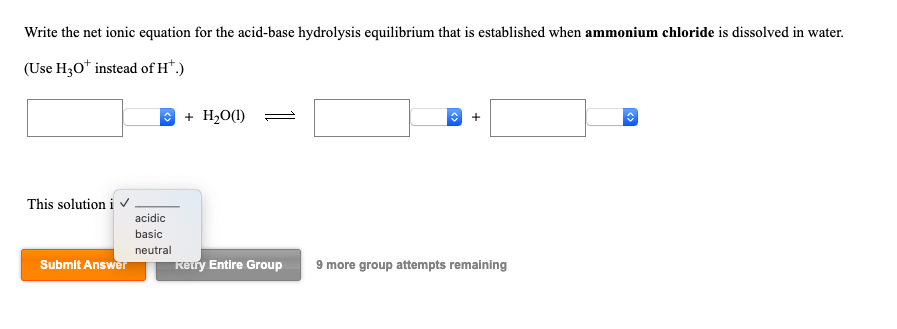
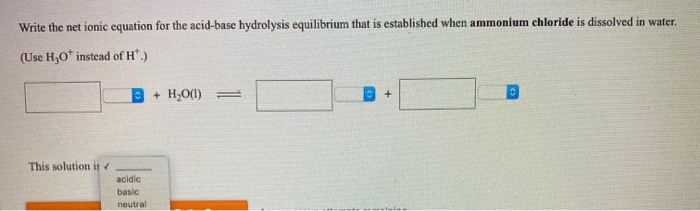
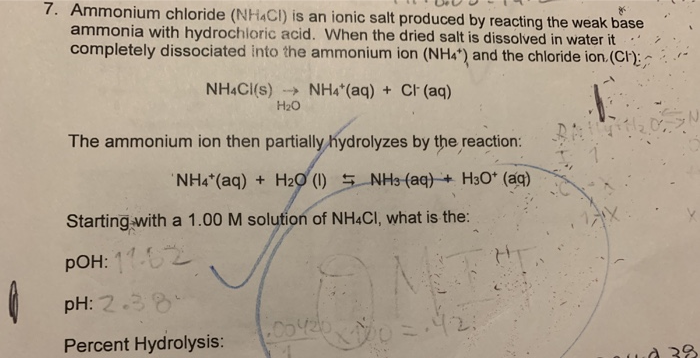SOLVED:A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.
Ammonium Chloride (NH4Cl) - Structure, Properties, Preparation, Uses, Health Risk & FAQs of Ammonium Chloride.

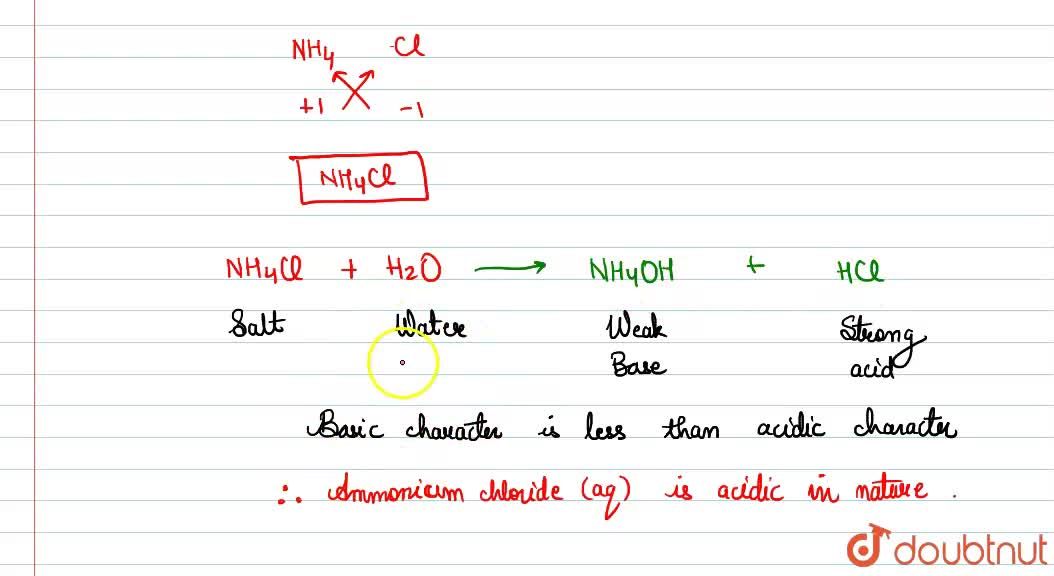
Identity the acid and base for ammonium chloride salt. state the nature of this salt and mention its - Brainly.in

SOLVED: In a blood buffer, ammonium chloride (NH4Cl) was added to lower the blood pH. The ammonium ion is what acts as the acid. The chloride ion does not have any acid/base

Question Video: Selecting the Correction Equation for the Reversible Reaction of Hydrogen Chloride and Ammonia to Make Ammonium Chloride | Nagwa