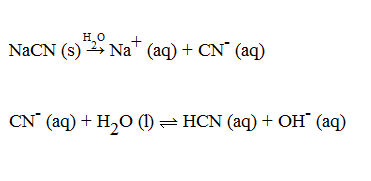What is the % hydrolysis of NaCN in N/80 solution when dissociation constant for HCN is 1.3 x 10^-9 & Kw = 1 x 10^-14 - Sarthaks eConnect | Largest Online Education Community

acid base - Why do we need three equations to find the pH of NaCN, given Ka(HCN)? - Chemistry Stack Exchange
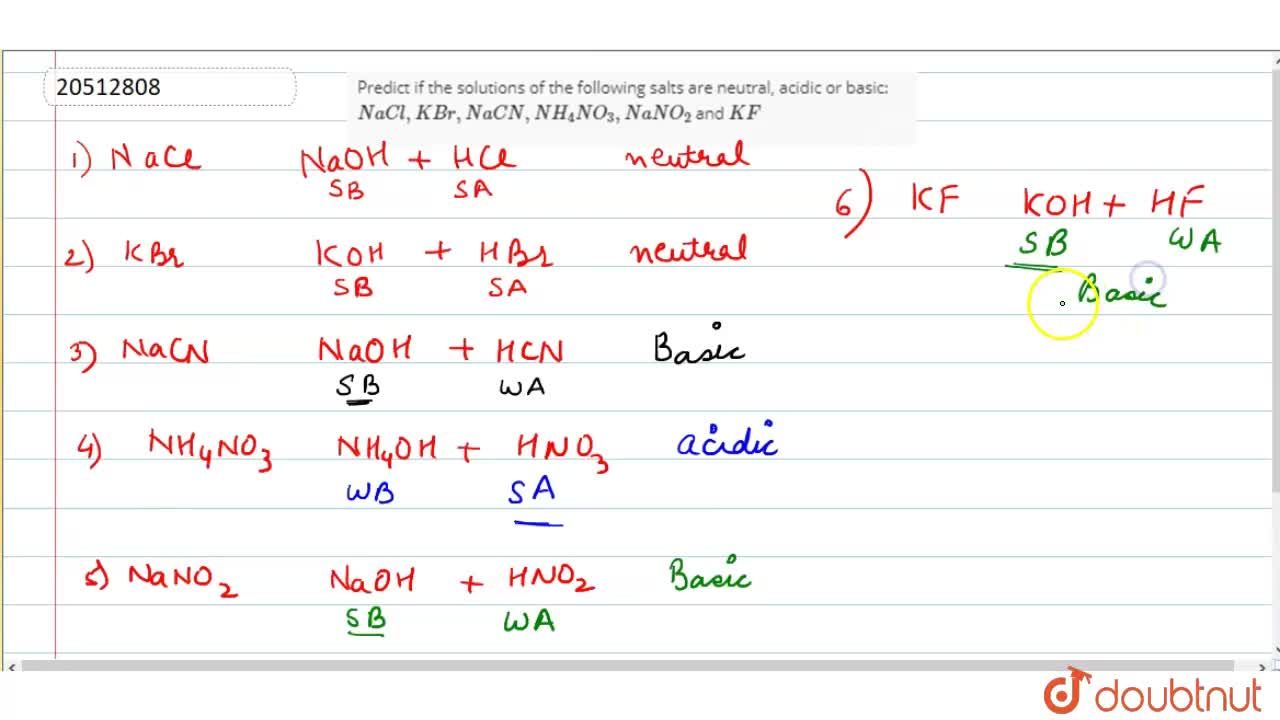
Predict if the solutions of the following salts are neutral, acidic or basic: NaCl, KBr, NaCN, NH(4)NO(3), NaNO(2) and KF

SOLVED: 16- Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 10 10). Which of the following statements is true? A) The solution is not
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...

Draw the major product formed in the following reaction with NaCN and other reactants ethanol and water. | Homework.Study.com

SOLUTION: NaCH3COO, HCl, HCN, NaOH, NH3, NaCN, KNO3, H2SO4, NH4Cl, H2SO3, NaHCO3, Na3PO4 and CH3COOH - Studypool
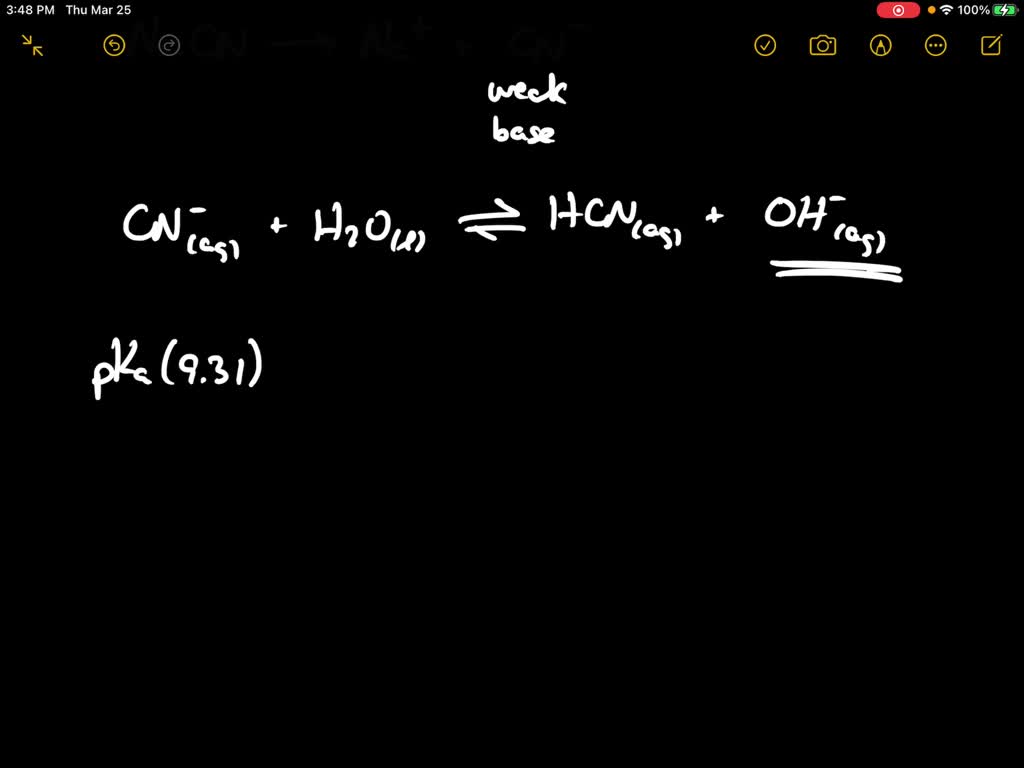
SOLVED:When NaCN dissolves in water, the resulting solution is basic. Account for this observation given that p Ka for HCN is 9.31.

Exam 3 Practice Problems - Sample Problems (Acid/Base and Solubility) CHM Determine if the following - StuDocu

Acids Lesson 6 Acid Rain & Hydrolysis. Acid Rain The cause of Acid Rain is the release of acid anhydrides into the environment. Acid Anhydrides are nonmetal. - ppt download