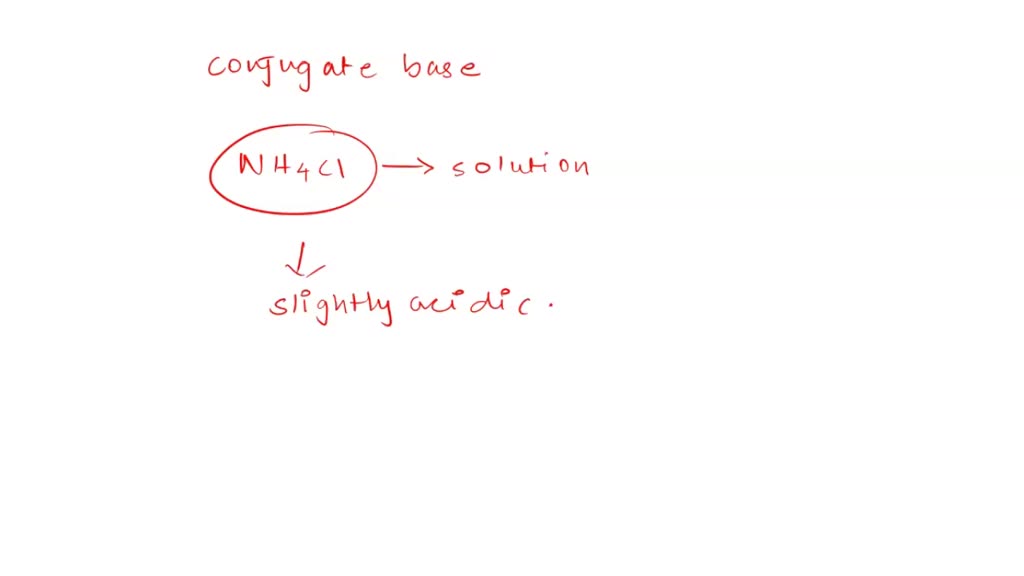
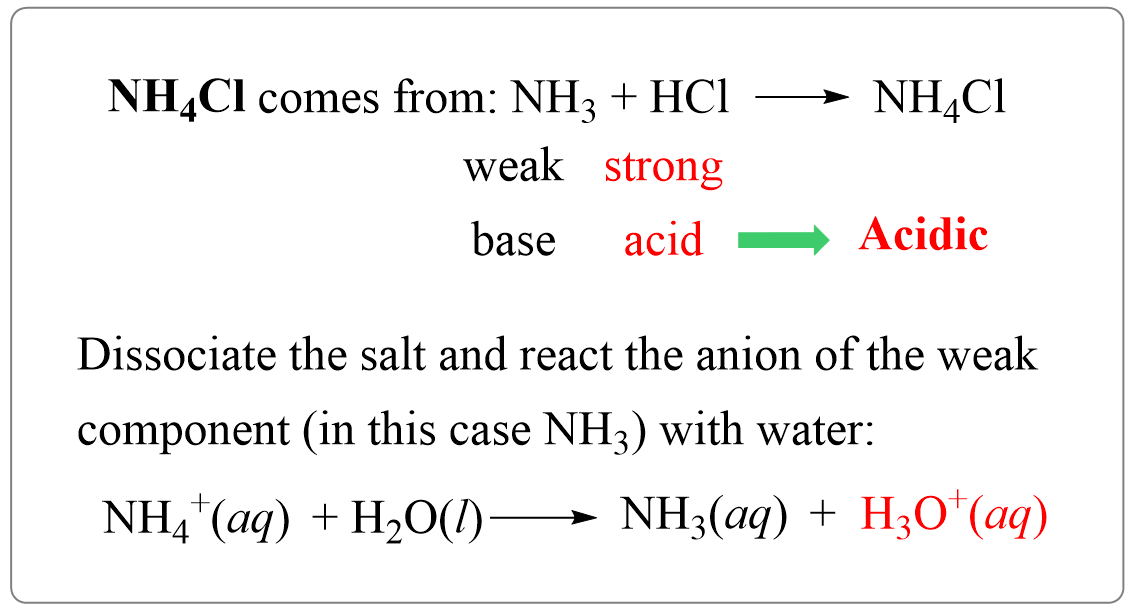
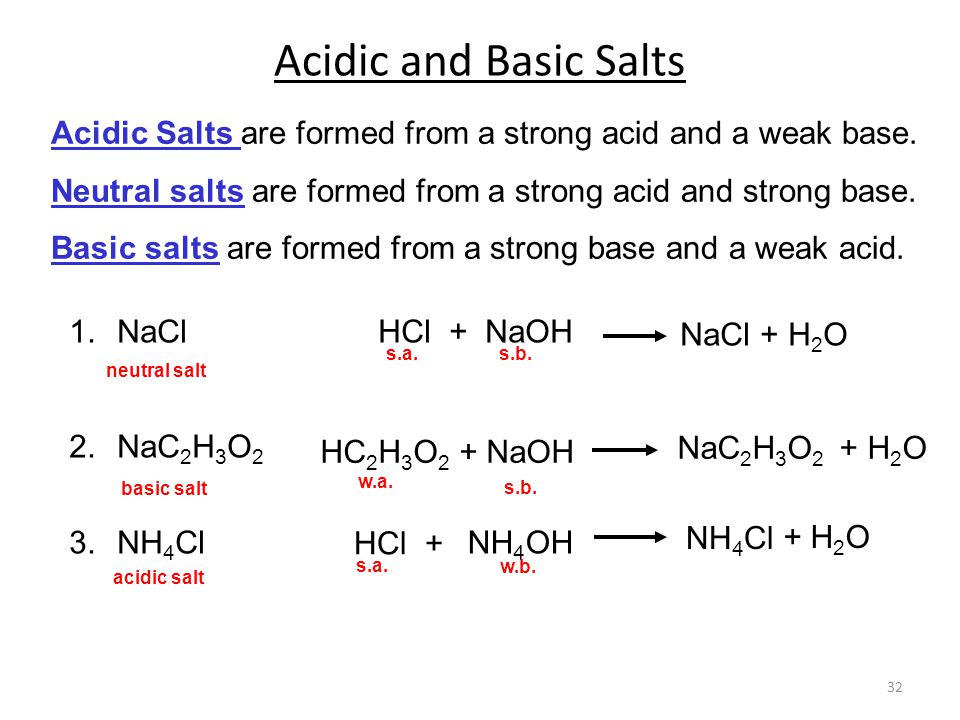
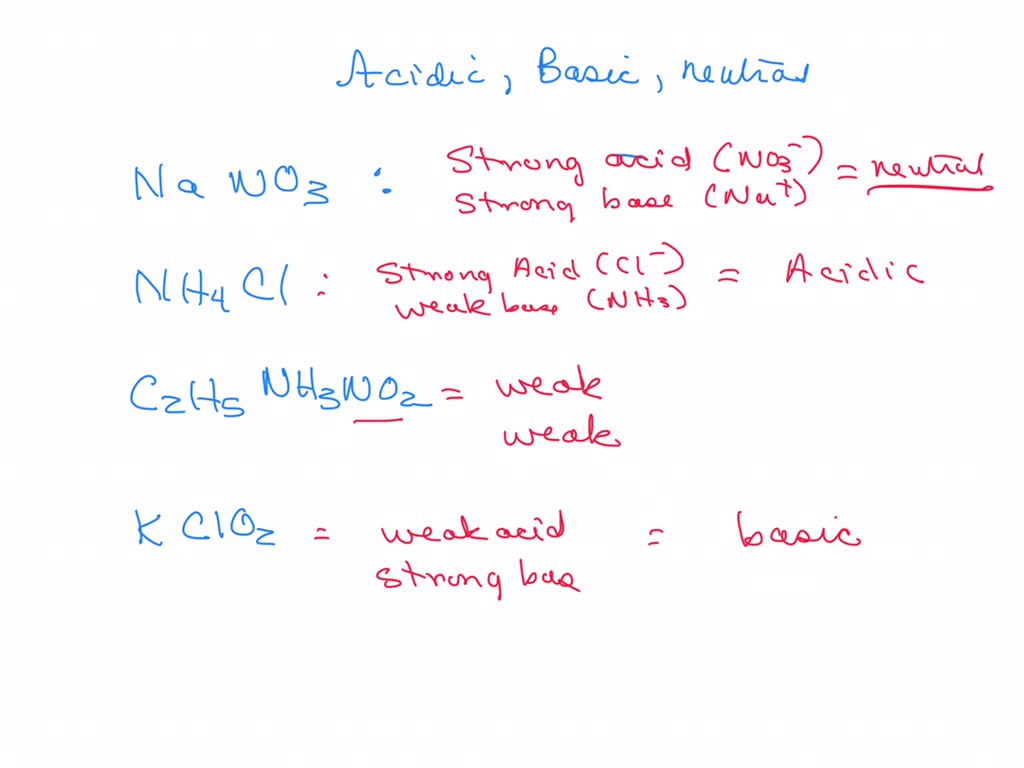
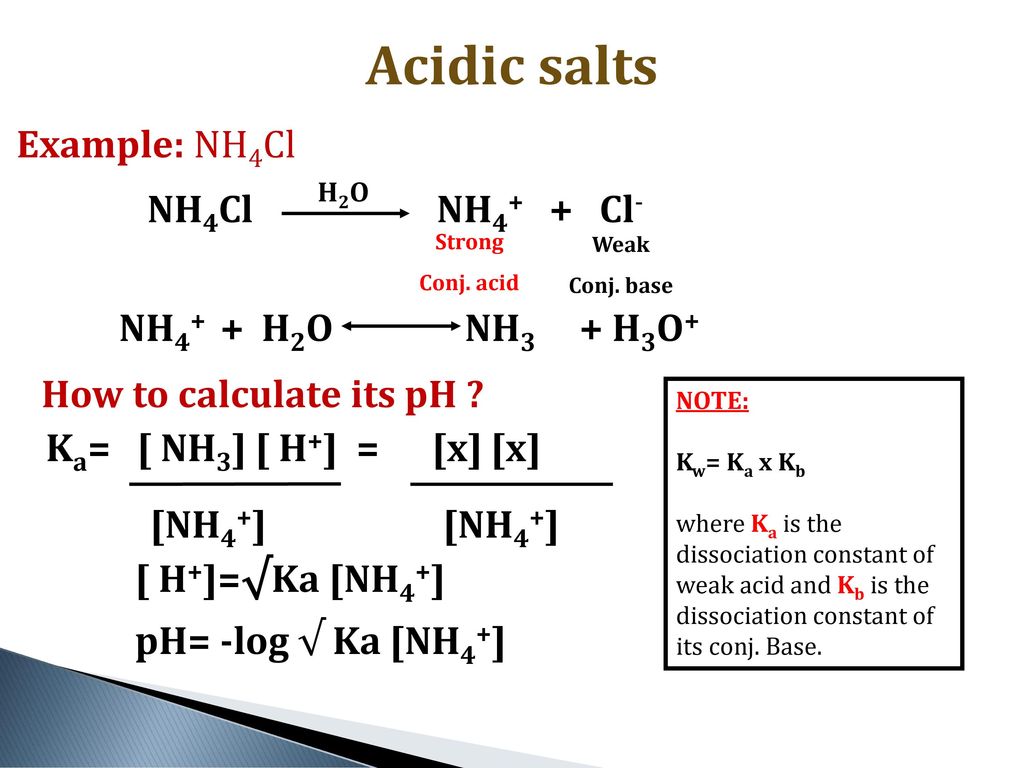
SOLVED: In a blood buffer, ammonium chloride (NH4Cl) was added to lower the blood pH. The ammonium ion is what acts as the acid. The chloride ion does not have any acid/base

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in

Question Video: Selecting the Correction Equation for the Reversible Reaction of Hydrogen Chloride and Ammonia to Make Ammonium Chloride | Nagwa